For decades it was assumed that protein channels and protein pumps fulfilled completely different functions and worked independently of each other. Researchers at Goethe University Frankfurt and University Groningen have now elucidated the transport path of a protein complex that combines both mechanisms: it first receives potassium from the channel and then transfers it to the pump, from where it is transported to the cell.
A balanced potassium household is critical for the survival of both people and bacteria. As bacteria are exposed to much greater fluctuations in environmental conditions, the controlled intake of potassium often poses a particular challenge. Since the cell membrane is impenetrable for potassium ions, it has to be translocated through specific membrane transport proteins.
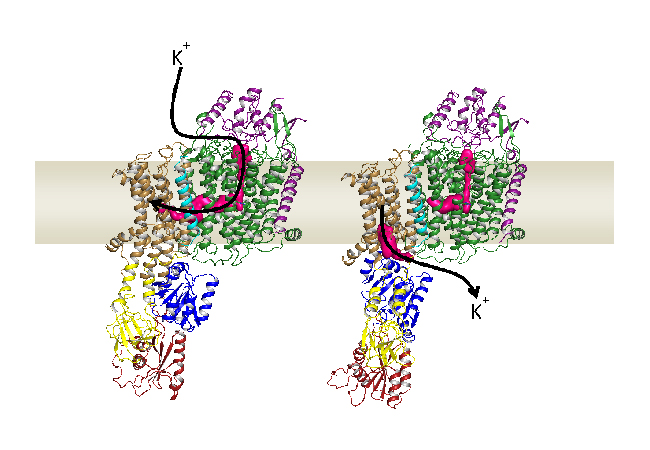
On the one hand, potassium channels enable the rapid, but passive influx of potassium ions. This stops as soon as an electrochemical equilibrium between the cell and its environment has been reached. To attain intracellular concentrations beyond this, potassium is transported into the cell actively through potassium pumps, with energy being consumed in the form of ATP.
Since both protein families – channels and pumps – carry out very different functions, they have always been described as separate from each other. This, however, is contradicted by the observation that KdpFABC, a highly affine, active potassium uptake sys-tem of bacteria, does not represent a simple pump, but is constructed of a total of four different proteins. One of these is derived from a typical pump, while another one re-sembles a potassium channel.
Inga Hänelt, Assistant Professor for biochemistry at Goethe University, and her colleague Cristina Paulino from University of Groningen, the Netherlands, therefore decided to take a closer look at the membrane protein KdpFABC through the microscope – or, more specifically, the cryo-electron microscope. They were surprised by the result: “All earlier hypotheses were wrong,” states Inga Hänelt. “Although we had all the data in front of us, it took us a while to understand the pathway potassium takes through the complex into the cell.”
First, a channel-like protein binds the potassium and transports it through the first tunnel to the pump. Once it has arrived, the first, outward-facing tunnel closes, while a second, inward-facing tunnel opens. This tunnel also extends between both proteins and ultimately ends in the interior of the cell. “The complex essentially combines the best qualities of both protein families,” explains Charlott Stock, doctoral candidate in Inge Hänelt’s research group. “The channel-like protein binds potassium, at first very specifically and with high affinity, while the pump enables an active transport that can enrich potassium in the cell by 10,000-fold.”
The data, recently published in Nature Communications, impressed the scientists with how diverse transport through membranes can be. “We have learned that when investigating various membrane transport proteins, we shouldn’t rely on seemingly incontrovertible mechanisms, but have to be ready for surprises,” summarises Inga Hänelt.
[dt_call_to_action content_size=“small“ background=“fancy“ line=“true“ animation=“fadeIn“]
Publication: Charlott Stock, Lisa Hielkema, Igor Tascon, Dorith Wunnicke, Gert T. Oostergetel, Mikel Azkargorta, Cristina Paulino, Inga Hänelt, Cryo-EM structures of KdpFABC suggest a K+ transport mechanism via two inter-subunit half-channels, in: Nature Communications
10.1038/s41467-018-07319-2
[/dt_call_to_action]
Source: Press release from 26 November 2018







