In the profile area Molecular & Translational Medicine, basic research and clinical practice are working closely together. Research fields include cardiology, oncology, neurosciences and pharmaceutical research. The focus lies on studying molecular pathophysiological processes and personalised treatment strategies. The aim of clinical translation is to integrate findings from basic research more quickly into patient treatment. A key goal is the development of innovative therapeutics. The increasingly important role of data science in medicine also fosters translational research.

The Excellence Cluster Cardio-Pulmonary Institute has conducted research into molecular processes that interact in the heart and lungs since 2006. Recently, the researchers have been working very closely with colleagues from clinical practice – for example on constricted heart valves.
The perfect interaction between heart and lungs is based on complex and precisely coordinated biomolecular processes. As long as they are in equilibrium, the body is always supplied with sufficient blood and oxygen. But what happens in individual cells and tissues when the heart and lungs are diseased? Since 2019, this has been one of the key questions explored by the researchers at the Cardio-Pulmonary Institute (CPI) – an excellence cluster in the current funding period of the Excellence Strategy of Germany’s federal government and states. Now as before, heart and lung diseases are the leading cause of death worldwide. Recently, the coronavirus pandemic has shown that many questions still need answering when it comes to treating these two vital organs.
The Cardio-Pulmonary Institute is now focusing increasingly on the transfer from basic research to clinical applications.
“Heart and lungs cannot be studied separately. It was therefore important for us as heart specialists to work closely with the lung researchers at the University of Giessen and the Max Planck Institute for Heart and Lung Research in Bad Nauheim,” explains Professor Stefanie Dimmeler, the cluster’s spokesperson. Collaboration began in 2006 with the Excellence Cluster Cardio-Pulmonary System (funded until 2019). During this time, a unique research network developed that specialises in the interaction of the two organs. The Cardio-Pulmonary Institute (CPI) is now focusing increasingly on the transfer from basic research to clinical applications. Alongside the five Discovery Areas dealing with special biomolecular processes, there are three Translational Hubs in which the researchers collaborate very closely with physicians: “Precision Medicine”, for example, which uses the findings of all research fields to develop new, individualised therapeutic approaches.
When mutant cells damage the heart
A current example of this type of interdisciplinary cooperation is the treatment of aortic valve stenosis – a narrowing of the aortic valve in the left half of the heart due to calcification. It occurs frequently from about 65 years of age. In contrast to atherosclerosis, the risk factors are unknown. Without surgical or catheter-based valve replacement, only half of those affected have a chance of surviving the next two years. It is not possible to predict which patients will most likely die and which would survive without surgery. In older or already weakened patients, the risk of surgery can now be reduced by inserting a new heart valve via a catheter and without opening the thorax. University Hospital Frankfurt is specialized in this TAVR procedure.
To be able to assess the risks for patients with aortic valve stenosis, knowledge is needed at cellular level: scientists already know that calcification of the aortic valve begins when a function of the endothelial layer on its surface is disrupted. This triggers a response from immune cells and inflammatory reactions occur. Ultimately, this leads to calcification of the heart valve tissue.
Another risk factor according to cardiologists is that the left half of the heart enlarges by pumping against the resistance of the narrowed valve. This leads to a pathological proliferation of connective tissue cells in the heart muscle (fibrosis). Cardiologists suspect that this is the reason why one in five patients nonetheless dies within three years after a successful valve replacement. Professor Andreas Zeiher is investigating the causes of this within an ERC Advanced Grant (CHIP-AVS), which was approved by the European Research Council in 2022.
The many years of cooperation between physicians and scientists in the cardiology and haematology/oncology priority programmes plays a central role in aetiology. It is already known from research conducted by haematologist Professor Michael Rieger that blood stem cells play an important role. These form in the bone marrow and can develop into red and white blood cells or platelets. Blood stem cells mutate with age. This provides a survival advantage, so that they multiply more. Since all blood cells in the body are constantly regenerating from stem cells in the bone marrow, these mutations also pass on to mature blood cells. Clones of mutant blood cells form, a process that has been termed “clonal haematopoiesis”. Some of these clones are risk factors for heart and lung diseases, including atherosclerosis, cardiac insufficiency, coronary heart disease, myocardial infarction and aortic valve stenosis.
For the first time, the group led by Michael Rieger has found clones with a specific gene mutation in the blood of patients with aortic valve stenosis. This gene encodes an enzyme with the abbreviation DNMT3A. “Mutations in DNMT3A are the most common drivers of clonal haematopoiesis,” he reports. Using state-of-the-art single cell sequencing, Professor Wesley Abplanalp’s group at the CPI was able to determine at cellular level that mutations of this gene lead to increased transcription of genes for pro-inflammatory signal substances.
Inflammation is thus exacerbated by clonal haematopoiesis. “This is presumably the reason why we often observe silent inflammation in older patients. It promotes disease and raises mortality,” says Michael Rieger. This is in line with the observation that immune cells and their messenger substances (cytokines) are involved in both valve calcification and pathological left ventricular hypertrophy.
“DNMT3A could cause the inflammatory reaction with subsequent calcification of the aortic valve and heart muscle fibrosis,” assumes Dr Silvia Mas-Peiro, head of the aortic valve project. For her colleague Dr Sebastian Cremer, who is leading the studies on heart failure, it is interesting that the mutant gene also occurs conspicuously often as a clone in his patients. There are many indications that it is a feasible marker for predicting the progression of aortic valve stenosis. “Once we’ve deciphered the disease mechanism, we can optimise treatment options based on improved risk assessment,” summarises Andreas Zeiher.
(ahv)
Excellence Cluster Cardio-Pulmonary Institute (CPI)
Joint project management: Goethe University Frankfurt
Institutional partners: Faculty of Biological Sciences and Faculty of Medicine of Goethe University Frankfurt, University of Giessen, Max Planck Institute for Heart and Lung Research, Bad Nauheim
Funding period: 1 January 2019 to 31 December 2025

We asked...
Stefanie Dimmeler
Which problem would you like to understand better?
We want to better understand, diagnose and treat heart and lung diseases. As you can see from the example of COVID-19, we are also repeatedly facing new challenges.
Why is this topic important?
Heart and lung diseases are unfortunately still the main causes of death.
What is your milestone?
To identify new therapeutic targets and possibly use them for the first time in humans.
What must not happen?
Resting on the laurels of past successes.
How do you deal with failure?
After I’ve calmed down, I try to learn from failure and to improve. My motto is: “Success makes you grow, failure makes you mature.”
How do you celebrate success?
Preferably down by the river with the team that contributed to the success.
Professor Stefanie Dimmeler is director of the Institute of Cardiovascular Regeneration at the Centre for Molecular Medicine and spokesperson for the cluster of excellence “Cardio-Pulmonary Institute”.
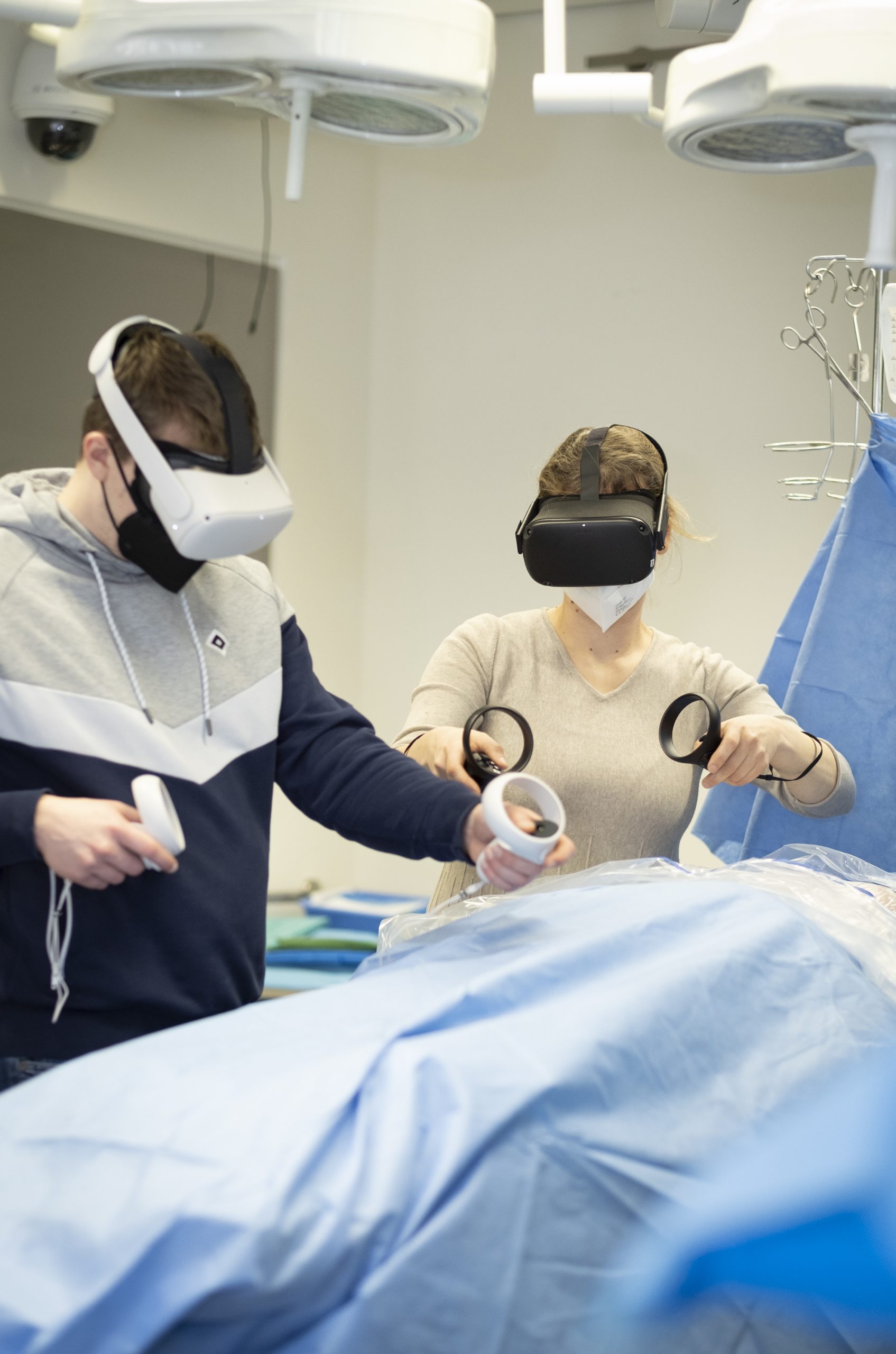
Most medical errors occur because communication fails. Miriam Rüsseler deals with this flaw in a creative way: in the Room of Error she has developed, healthcare professionals learn to work as a team.
A hazelnut yogurt on the bedside table, the walking frame in a corner of the room, in the middle a patient unable to move. This is the scenario that presents itself to prospective medical students and nursing staff as soon as they put on the Room of Error virtual reality (VR) goggles. Spot the mistake: only those with a trained eye for finer details and the seemingly insignificant will discover things in the virtual hospital room that could endanger the patient’s life (does it say anything about nut allergies in their medical records?).
According to studies, 70 percent of all medical errors are due to human error, explains Professor Miriam Rüsseler, physician and qualified medical education expert at University Hospital Frankfurt. To train attention, especially under stress, to develop a culture of error and to overcome invisible barriers between the job of the nurse and that of the doctor: this is the goal of the Room of Error she has developed for prospective physicians and nursing staff.
(No) gap in the curriculum
As head of Interdisciplinary Simulation Training, she reacted quickly during the pandemic: after the real Room of Error was no longer accessible, she had a virtual simulation programme including VR goggles developed at short notice (also funded by the Goethe Corona Fund). The advantage is that participants can shift from wherever they are to an emergency room or a hospital ward, learn about the case there – and then exchange their observations. “The actual learning effect lies in the team discussion,” Miriam Rüsseler has discovered.
While still a medical student, this gap in the curriculum bothered her, which is why she has devised projects to improve medical teaching – and invested prize money in her own didactic training. In 2019, she then launched the teaching project “Cutting Through Barriers”, with interprofessional shock room training at the learning centre of University Hospital Frankfurt. However, the aim is for not only the around 100 surgery students to experience the Room of Error but everyone training at University Hospital. Rüsseler is currently recruiting additional instructors for this.
What motivates her: “Passion” is her spontaneous answer. She greatly enjoys supporting interprofessional teams. That she sometimes runs into a brick wall with her project in the hospital’s hierarchical daily routine only slows her down for a moment. Her success shows that she is on the right track: in the summer of 2022, Miriam Rüsseler is to be appointed as the new Professor for Medical Education and Clinical Simulation at Goethe University Frankfurt and University Hospital Frankfurt. This is linked to the establishment of an Academic Health Centre – where team training for emergencies will play a prominent role.

The cluster project TRiC (Targeting Resistance in Cancer) is investigating why cancer cells are resistant to therapies. And which drugs can change this. This is why researchers and clinicians are working closely together.
Therapy resistance is the most common cause of death in cancer. Some cancer cells are already resistant before treatment, others develop resistance during treatment. There are many causes for this: they might lie in the tumour cell itself or in the surrounding tissue. They might be influenced by external factors such as infections, or by a patient’s lifestyle.
In a network of experts from medicine, chemistry, pharmacy and informatics, the researchers in the TRiC cluster of excellence (Targeting Resistance in Cancer) want to unravel the complex causes of resistance. TRiC is based on three pillars: 1. understanding therapy resistance in cancer and finding targets for new drugs; 2. developing and testing drugs; 3. understanding complex correlations with artificial intelligence.
The therapy for rectal cancer is one example that illustrates how important it is to understand resistance. It is successful in many cases: in recent years, a new treatment regimen, a combination of radiotherapy and chemotherapy, has been developed that can eliminate the need for surgery. “However, there are always patients who do not respond or respond insufficiently to standard chemoradiotherapy,” explains Professor Claus Rödel, Director of the Department of Radiotherapy and Oncology. Together with Professor Emmanouil Fokas, he heads the German Rectal Cancer Study Group, which is investigating this new kind of therapy.
When cancer cells meet AI
To better understand the resistance mechanism, the physicians turned to Professor Florian Greten. He is the spokesperson of the Frankfurt Cancer Institute (FCI), a LOEWE centre, where interdisciplinary teams investigate the molecular mechanisms of cancer development and then derive individual therapies. When examining tissue and blood samples, the researchers made a surprising discovery: it is not the tumour cells that are the decisive factor for resistance, but the surrounding connective tissue cells, altered by inflammation (i.e. the tumour microenvironment).
In animal experiments, the researchers at the FCI were able to show that by inhibiting a certain pro-inflammatory messenger substance the cancer becomes susceptible to radiation again. The anti-inflammatory drug is being tested in cancer patients in a clinical trial.
The researchers also discovered that patients whose blood serum contains specific receptors for an inflammatory messenger substance are more likely to respond to chemoradiotherapy. “This also enables us to predict which patients will benefit from an accompanying anti-inflammatory therapy,” explains Dr Adele Nicolas, a scientist from Georg-Speyer-Haus and first author of the study. In the TRiC cluster project, this approach will now be extended to different types of cancer.
There has been long-standing expertise in cancer research in Frankfurt since the founding of the University Cancer Centre (UCT) in 2009. Another milestone for translational research, i.e. the close exchange between basic researchers and clinicians, was the founding of the Frankfurt Cancer Institute in 2019. Since 2017, Goethe University Frankfurt has been one of the main sites of the Structural Genomics Consortium (SGC), which currently coordinates the collaboration of about 200 scientists at renowned academic institutes and eight pharmaceutical companies. And it is thanks to the establishment of the SGC that a targeted search for active substances in databases is possible and that these active substances are made publicly available to scientists. Moreover, the Structural Genomics Consortium initiated the European network “EUbOPEN” led by Professor Stefan Knapp at Goethe University Frankfurt: its goal is to develop a substance library of small, highly selective chemical modulators that alter protein function and thus contribute to the systematic elucidation of fundamental biological and disease-related processes.
"AI systems trained with expert knowledge are already able to reliably detect skin cancer."
Kristian Kersting
A new addition to the cluster of excellence project is artificial intelligence (AI). “AI systems trained with expert knowledge are already able to reliably detect skin cancer,” explains Kristian Kersting, Professor of Artificial Intelligence and Machine Learning at the Technical University of Darmstadt, with which Goethe University Frankfurt is collaborating within the alliance of Rhine-Main Universities (RMU). What computer scientists in the TRiC project are now aiming for is far more complex. They want to link clinical data, such as lab values and MRI or ultrasound scans, with information about the genes and proteins of individual patients. In recent years, analysing genetic defects in cancer has made it possible to increasingly individualise (chemo)therapies. Protein defects have now come into focus. These can be used, for example, as specific molecular markers for diseases and represent a further step towards individualised therapy.
To be able to recognise new correlations and factors for resistance in the vast amounts of data that can nowadays be collected for individual patients, artificial intelligence is needed. That is a challenge for AI researchers like Kersting because he has to “teach” the system to look for something that human experts cannot tell it to look for. And once the system has found a correlation, it must be able to “answer” testing questions from a human. “If we apply AI to biomedical systems, we will have to develop completely new methods that will take the entire field of AI a big step forward,” says Kersting.
(ahv)
TRiC
Project management:Project management: Goethe University Frankfurt (speaker university), Technical University of Darmstadt
Participating partner institutions:Georg Speyer Haus (GSH); Max Planck Institute for Heart and Lung Research, Bad Nauheim; Max Planck Institute for Terrestrial Microbiology, Marburg; Paul Ehrlich Institute; Frankfurt Blood Donation Centre; LOEWE research initiative Frankfurt Cancer Institute (FCI); University Cancer Centre (UCT); German Cancer Consortium (DKTK); Structural Genomics Consortium (SGC); Hessian Centre for Artificial Intelligence (hessian.AI); IMI (Innovative Medicines Initiative); EUbOPEN project; LOEWE priority programme TRABITA.

We asked...
Hubert Serve
Which problem would you like to understand better through the TRiC research project?
We want to understand why some patients respond better to cancer therapies than others.
What is important about it for you personally?
At the present time, we are experiencing that many new drugs and methods are flooding the market. The result is a wide range of possible combinations. We want to identify the best combination for each individual – but traditional methods of examining patients are no longer sufficient for this.
What is your milestone?
We would like to demonstrate reliable solutions for groups of patients that are as clearly defined as possible in a respective situation in order to overcome therapy resistance.
What is the biggest obstacle?
The biggest obstacle is understanding the complexity of the signalling pathways that lead to therapy resistance. We will use new, computer-aided methods, such as artificial intelligence (AI), for this difficult question.
How do you deal with failure?
We act according to the principle: “Try harder”. Unfortunately, in medicine we are accustomed to having to pursue many different approaches until one finally works. But basically, failure is an incentive.
How do you celebrate success?
When I look into the eyes of patients who are alive because we were able to leave the beaten track together with them and new therapeutic approaches were successful, then that is the greatest success for me.
Professor Hubert Serve is in charge of the Department of Medicine II at University Hospital Frankfurt and principal investigator (PI) in the TRiC research project.
Unser Forschungsprofil: Raum für gute Antworten
Mit sechs Profilbereichen will die Goethe-Universität ihre Kompetenzen stärker bündeln.

