This profile area aims to understand life processes in order to develop novel therapies and efficient biotechnological applications as well as describe dynamic interdependencies in ecosystems. To this end, molecular processes, cellular architectures and the behaviour of organisms are studied experimentally and theoretically, using modern methods from all natural and life sciences, many of which were developed in Frankfurt. Research foci include RNA-based processes, membrane and organelle dynamics, neuronal molecular and cellular architectures, cellular and cell-cell interactions, and light-driven processes.
Many diseases are driven by extremely complex processes. How, for example, do viruses and bacteria interact with the human body? How do inflammatory reactions evolve? What happens when the cellular balance tips? To develop new therapeutic strategies, the ENABLE cluster project is investigating these processes.
To keep the human body in functional shape and to successfully fend off threats, a vast communication network needs to be active. To achieve this, cells rely on many different quality control mechanisms to maintain their internal balance – also called “homeostasis” by scientists. For example, cells are capable of detecting and repairing potential damage, removing superfluous components and bringing dysregulated signalling pathways back under control.
If cells fail to maintain their equilibrium, the consequences are severe: dysregulated cell division, for example, is a cause of cancer. Hyperactive signalling pathways can trigger chronic inflammatory processes. And misfolded proteins that accumulate in cells play a central role in neurodegenerative diseases such as Alzheimer’s dementia, Parkinson’s disease and amyotrophic lateral sclerosis (ALS).
So how do cells monitor their balance? How do they react to viral and bacterial threats? What factors trigger and fuel chronic inflammation and how can such a response be controlled? How can innovative tools be used to modulate such complex molecular events? These are questions that the cluster project ENABLE (Unravelling mechanisms driving cellular homeostasis, inflammation and infection to enable new approaches in translational medicine) is exploring in three complementary research areas.
To identify approaches for novel drugs, ENABLE researchers are particularly interested in the complex interplay of signalling pathways that monitor cellular homeostasis, control immunological responses and defend against threats.
Key quality control mechanisms include the ubiquitin-proteasome system and the autophagy-lysosome pathway - two systems that serve to degrade damaged or superfluous cellular components. Professor Maike Windbergs from the Institute of Pharmaceutical Technology at Goethe University Frankfurt, spokesperson for the ENABLE project, explains: “We will investigate the molecular and spatial organization of the underlying processes with hitherto unknown precision. Above all, we wish to understand how the different cellular signalling networks are coordinated on a higher level. There are several obvious links between inflammatory responses and cellular quality control systems. This is highly exciting because it opens up new possibilities for modulating cellular responses".
In a second large thematic block, ENABLE researchers are studying how bacteria and viruses interact with human cells, which immune responses are triggered and how tissue damage and diseases evolve. Similar to the SARS-CoV-2 spike protein enabling infection of human host cells, all viruses and bacteria come with a set of specific molecules facilitating host cell or tissue penetration or evasion of the immune system. “A precise understanding of these mechanisms is needed to develop effective drugs against antibiotic-resistant bacteria or emerging viruses such as SARS-CoV-2,” says Professor Ivan Đikić from the Institute of Biochemistry II at Goethe University Frankfurt, spokesperson for the ENABLE project.
„Today we know that inflammation and the corresponding immune responses can also cause and contribute to complex diseases.“
Maike Windbergs
Finally, the third thematic block of the ENABLE project focuses on inflammatory reactions. Historically, inflammation has been viewed solely as the body’s protective response to injury and invading pathogens: damaged cells or cells attacked by viruses release signals which in turn attract and activate immune cells, thereby initiating molecular defence mechanisms. “Today,” says Windbergs, “we know that inflammation and the corresponding immune responses can also cause and contribute to complex diseases such as cancer, heart conditions and Alzheimer’s. By deciphering at the cellular and molecular level how inflammation arises and is resolved, we are laying the foundation for new therapeutic strategies. We aim to develop promising approaches for inflammation therapy, which may also have to be tailored more closely to individual patients.”
All three ENABLE topics - homeostasis, infections and inflammation - are closely intertwined via cellular signalling chains. As Đikić says: “That is why we are collaborating across disciplines, using cutting-edge technologies and novel, chemical and biological tools that allow us to analyze cellular functions with unprecedented precision." Innovative platform technologies are a central component of ENABLE and crucial for the success of the ambitious research projects. Substantial support for the development of such technologies is also provided by the Cluster4Future PROXIDRUGS, which prevailed in a federal competition in 2021 and is funded with up to €15 million. The novel class of proximity-inducing drugs allows the targeted degradation of disease-relevant proteins and thus opens up new therapeutic options for various diseases.
The ENABLE cluster project builds on existing strong research networks and technology platforms at Goethe University Frankfurt, the Max Planck Institute of Biophysics, the Frankfurt Institute for Advanced Studies, Georg-Speyer-Haus and the Fraunhofer Institute for Translational Medicine and Pharmacology. In the future, the network will be extended even further: for the EMTHERA (Emerging Therapeutics) initiative, ENABLE has forged a powerful alliance with colleagues at Johannes Gutenberg University Mainz. At both locations, a large number of faculties – from medicine to chemistry and life sciences to economics – are working together in an interdisciplinary manner.
(mbe/pb)
ENABLE
Goethe University Frankfurt (speaker), Max Planck Institute of Biophysics, Frankfurt Institute for Advanced Studies, Georg-Speyer-Haus and Fraunhofer Institute for Translational Medicine and Pharmacology
Partner institutions involved:
Faculties of Economics/Business, Computer Science/Mathematics, Biochemistry/Chemistry/Pharmacy, Biological Sciences, and Medicine, Max Delbrück Centre (Berlin), Max Planck Institute for Heart and Lung Research (Bad Nauheim), Max Planck Institute of Molecular Cell Biology and Genetics (Dresden)
Networking for new therapies


We asked...
Maike Windbergs and Ivan Đikić
Which problem would you like to understand better through ENABLE?
Maike Windbergs: Through ENABLE, we want to learn to understand better the interfaces between infection, inflammation and immune responses in order to develop new approaches for diseases that are not yet curable in an adequate manner.
Ivan Đikić: COVID-19 is a good example here – severe courses of the disease are often accompanied by an excessive immune reaction, where the immune system ultimately attacks and destroys the body’s own tissue.
Ivan Đikić: COVID-19 is a good example here – severe courses of the disease are often accompanied by an excessive immune reaction, where the immune system ultimately attacks and destroys the body’s own tissue.
What is important about it for you personally?
Windbergs: Even though we are both working in basic research, our training as physician and pharmacist, respectively, was guided by our aspiration to solve some of the greatest biomedical challenges of our times. This can only be achieved in an interdisciplinary consortium, which is why it’s important to assemble a team of different specialists in order to find completely new research approaches.
Đikić: This project enables us to further expand the platform technologies already available and to use them in such a way that new findings can be rapidly transferred into a clinical context. Apart from the fact that we are making an important contribution to new therapeutic strategies with this, we are also making a significant contribution to training a new generation of interdisciplinary researchers for the future.
Đikić: This project enables us to further expand the platform technologies already available and to use them in such a way that new findings can be rapidly transferred into a clinical context. Apart from the fact that we are making an important contribution to new therapeutic strategies with this, we are also making a significant contribution to training a new generation of interdisciplinary researchers for the future.
What is the biggest obstacle?
Windbergs: A major challenge, of course, lies in understanding the interfaces and interaction between different mechanisms in the human body and in developing suitable tools and models – for example, 3D models from human tissue. With such models, we systematically mimic the situation in the human body in order to enable transferability to clinical practice. In this way, we can, for example, develop targeted strategies for highly effective antibiotics and antivirals.
Đikić: At the end of the day, however, we never know all the obstacles that will stand in our way in a project. To a certain extent, that is also the special scientific appeal – we are moving in new territory, and there are always surprising discoveries and equally unexpected challenges. Having the right team and the right infrastructure is therefore essential so that we can react flexibly and quickly to new findings.
Đikić: At the end of the day, however, we never know all the obstacles that will stand in our way in a project. To a certain extent, that is also the special scientific appeal – we are moving in new territory, and there are always surprising discoveries and equally unexpected challenges. Having the right team and the right infrastructure is therefore essential so that we can react flexibly and quickly to new findings.
Have you discovered anything that has particularly influenced you?
Windbergs: A formative experience for all of us was certainly how the platform technology developed in Mainz for the production of mRNA-based vaccines led to the development of effective COVID-19 vaccines in record time. As far as my own laboratory is concerned, the special thing for me is our successful replication of viable 3D human tissues, which we can also use to simulate diseases. These will be extremely valuable in our envisaged projects for exploring mechanisms and validating therapeutic strategies in the context of tissue.
Đikić: A few years ago, my laboratory made a groundbreaking discovery when we successfully shed light on a previously unknown type of ubiquitin modification through Legionella enzymes. Using a whole repertoire of structural and functional studies enabled us to explain and understand both molecular details as well as the processes involved in the cellular context of a Legionella infection. This broad spectrum of methods, which we are meanwhile also combining with bioinformatics methods, for example dynamic modelling, has become an indispensable tool for the future. With our PROXIDRUGS cluster, we have also laid the foundation for the first time for the development of molecules that could also be used in therapeutics.
Đikić: A few years ago, my laboratory made a groundbreaking discovery when we successfully shed light on a previously unknown type of ubiquitin modification through Legionella enzymes. Using a whole repertoire of structural and functional studies enabled us to explain and understand both molecular details as well as the processes involved in the cellular context of a Legionella infection. This broad spectrum of methods, which we are meanwhile also combining with bioinformatics methods, for example dynamic modelling, has become an indispensable tool for the future. With our PROXIDRUGS cluster, we have also laid the foundation for the first time for the development of molecules that could also be used in therapeutics.
Professor Maike Windbergs and Professor Ivan Đikić are spokespersons for the ENABLE research project. Pharmacist Windbergs is professor for animal welfare at the Institute of Pharmaceutical Technology, molecular biologist Đikić is director of the Institute of Biochemistry II.
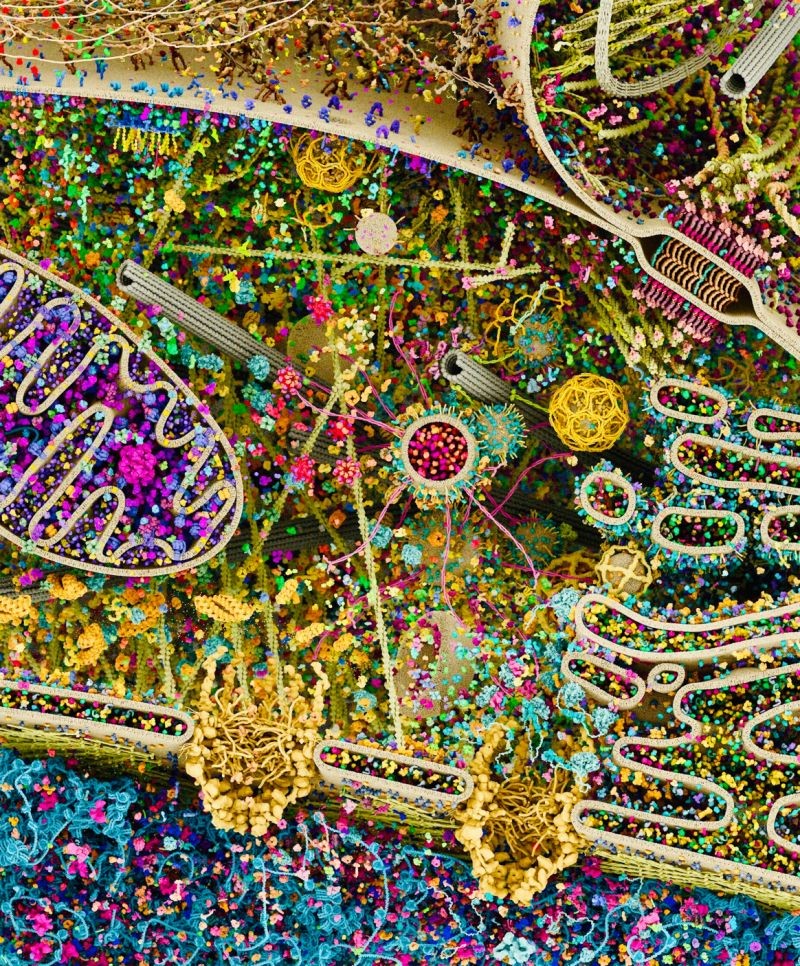
Thanks to modern imaging techniques, it is now possible to study cells down to the atomic scale. In the SCALE cluster project, recent technological advances should allow a better understanding of diseases.
Shedding light on the structures of RNA and protein complexes has traditionally been a key strength of scientists on Riedberg Campus. “We want to build on this strength with SCALE,” says biologist Michaela Müller-McNicoll, who, together with biophysicist Achilleas Frangakis, is the speaker for the interdisciplinary cluster SCALE (“Sub Cellular Architecture of Life”). The aim of the cluster project is to look more closely at the processes taking place within the cells of a living organism. So closely that by using state-of-the-art imaging techniques it is possible to visualise individual molecules and even individual atoms and follow their dynamics under different circumstances. “We want to observe how a molecule’s environment influences it and how individual molecules function and interact with each other in a completely crowded cell,” she says.
„We want to research how molecules interact in a densely packed cell.“
Michaela Müller-McNicoll
“You can think of a cell as if it was a house,” explains Müller-McNicoll, “just as the house has a kitchen, a bathroom, a living room and a bedroom and perhaps a storeroom, the cell also has several compartments or organelles: cell nuclei, mitochondria, ribosomes or synapses, which are the contacts between nerve cells.” The rooms are surrounded by walls, she says, and things can be transported in and out of doors or windows. “And just as you can shift a room divider or move a table from one corner to the other, the cell also additionally has dynamic architectural elements that form or dismantle as circumstances change,” she continues. Müller-McNicoll, Frangakis and the other researchers participating in SCALE therefore want to observe how the status quo changes and ultimately find out how the components combine to form larger functional architectural elements. In the long term, the aim is to explain how these changes influence the dynamic state of equilibrium in a living cell: whether a cell under stress reacts with “architectural changes” and how these affect the individual cell and the surrounding tissue.
Müller-McNicoll lists three objectives that are to be achieved through work in the SCALE project: “On the one hand, we want to create dynamic models: that is, to model all the parts of the cell in the computer so that we can predict which ‘remodelling’ measures are undertaken by cells reacting to stress, for example to extreme temperatures or to a viral infection.” A second objective is to apply the principles of construction and degradation processes that shape subcellular architecture to create novel synthetic organelles that produce new proteins, possibly even drugs. “And finally, we hope that SCALE will contribute to understanding – and ultimately curing – diseases that have to do with pathological changes in subcellular architecture.” This is assumed to be the case in neurodegenerative diseases such as Parkinson’s and Alzheimer’s, in which insoluble structures form in the cell plasma, which then completely clog the cell.
When cells remodel themselves
Structures of this kind, plaques of the protein building block amyloid-beta (Aβ), deposit themselves, for example, in the brain of Alzheimer’s patients. The project “Subcellular Architecture of the Vascular System in Health and Disease” by chemist Professor Nina Morgner and molecular biologist Professor Jasmin Hefendehl is studying these structures. Morgner uses the analysis methods “ion mobility spectrometry” and “mass spectrometry” to investigate how Aβ building blocks of different lengths join together to form plaques. The toxicity of the Aβ molecules depends in particular on their length. “Especially Aβ42, in which 42 basic building blocks are connected to each other, has a particularly harmful effect. We consider Aβ40 to be much more harmless,” explains Morgner. Understanding in detail how the protein building blocks combine to form Aβ plaques also opens up the possibility of intervening in this process. This in turn would substantially advance the search for an active ingredient for a drug against Alzheimer’s.
Hefendehl’s research on the toxic effect of Aβ plaques begins at a slightly different point: she brings Aβ chains of different lengths into contact with precisely that type of cell that makes up blood vessels in the brain. Together with Achilleas Frangakis, she then observes which pathological changes occur in the cells. “In over 80 percent of Alzheimer’s patients, electron microscopy reveals blood vessel disorders,” says Hefendehl. She suspects that the blood-brain barrier is defective in these patients. In this case, the brain would be defenceless against the toxic effect of the inflammatory proteins in the blood.
It is recent technological advances that have made the SCALE experiments possible in the first place: “Our cluster is benefiting significantly from the fact that cryo-electron microscopy, that is, electron microscopy at low temperatures such as almost minus 200 °C, has made enormous progress over the past ten years,” explains Müller-McNicoll. With this method, it is now possible to study molecules in cells and cell membranes at atomic resolution – in their native environment: although the subcellular structures are frozen, the researchers can observe subcellular architecture without having to purify, isolate or otherwise pre-treat them.
The SCALE cluster’s three fundamental themes require an interdisciplinary approach: the leading scientists come from physics and biology as well as chemistry, biochemistry and pharmacy. “We are very pleased that the Max Planck Institute of Biophysics and for Brain Research as well as the Frankfurt Institute of Advanced Studies are working together with us in SCALE,” says Müller-McNicoll. “This means that many of the institutes based on Riedberg Campus are involved in our project.”
(she)

We asked...
Michaela Müller-McNicoll
Which problem would you like to understand better through SCALE?
SCALE deals with subcellular architecture, that is, all the larger structures in a cell that either define a specific space or perform a specific function. These include organelles such as the mitochondrion, contacts between cells, for example synapses or desmosomes, or nuclear pores. Within SCALE, we want to understand at the molecular level how these subcellular structures emerge, how they are organised, how they are degraded, which functions they perform and how they adapt to changing conditions.
What is important about it for you personally?
I study the role of various structures in the cell nucleus, also referred to as nuclear architecture, in the processing of mRNA. These structures emerge or change when cells are stressed or diseased, and I want to understand their impact on gene expression.
What is your milestone?
We want to develop methods and technologies to observe activities inside the cell with high spatial and temporal resolution, manipulate them locally and assign them to a certain function.
What is the biggest obstacle?
The technologies are very complex and expensive and can only be financed and managed within large consortia.
Have you discovered anything that has particularly influenced you?
Interdisciplinary collaboration and the use of artificial intelligence are essential for studying and understanding increasingly complex interdependencies within cells.
Professor Michaela Müller-McNicoll is head of RNA Biology at the Institute for Molecular Biosciences and spokesperson for the SCALE research project, together with Professor Achilleas Frangakis.

Observing, examining, studying under the microscope – prospective biology teachers too are reliant on laboratories during their studies. So what do you do if you can’t study “inside” due to the pandemic? “Online” is the answer – or “outside”.
How can you make schoolchildren enthusiastic about plants? In didactics seminars, prospective biology teachers learn how to plan lessons that are appropriate for the children’s age and interests. In the seminars, students test, experience, conceptualise and contemplate beforehand how schoolchildren gather biological knowledge, how they can conduct experiments, and how they handle microscopes. Usually on site, in the university’s rooms. During the pandemic, it became apparent that some elements of a didactics seminar can also be done at home. Build a model of a skeletal muscle? Test thermal insulation? It can be done – with a little imagination and some everyday materials, such as pipe cleaners and feathers or animal hair wrapped around a container with warm water, biological knowledge can be taught in a vivid way using models and then shared with others via photos and videos for subsequent discussion.
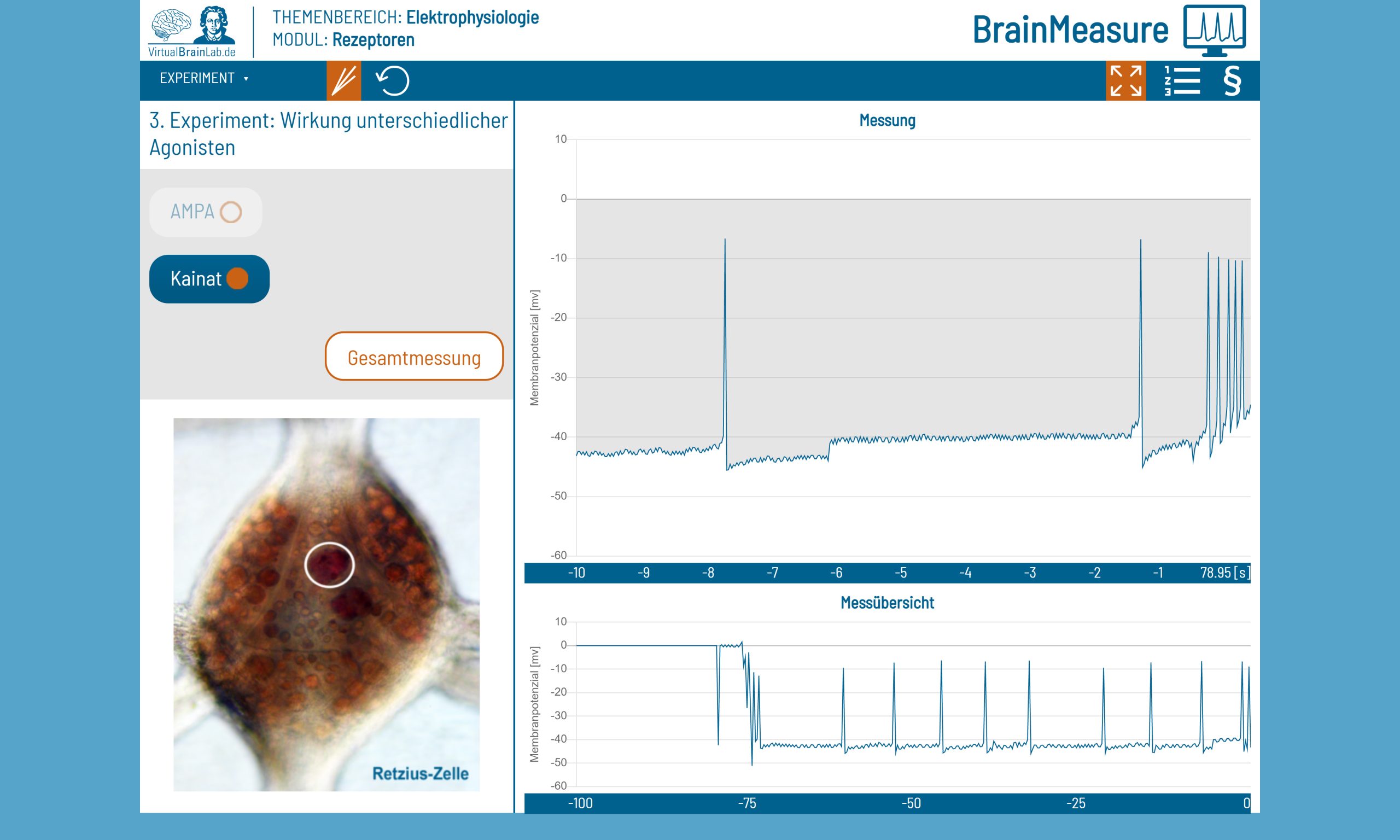
When model building reaches its limits, digital descriptions of experiments, video documentation and online materials are helpful. For example, the contents of the practical course “Human Biology and Anthropology” were taught via “VirtualBrainLab.de”, an online school laboratory for neuroscience. The VirtualBrainLab was developed at the end of 2020 by the Department of Didactics in the Biological Sciences and Zoo Biology at Goethe University Frankfurt for use in schools and then adapted to university courses. Original data formed the basis for virtual electrophysiological experiments and reactions of nerve cells that could be followed live. By using a virtual microscope, students were also able to compare healthy and pathological blood cells.
Incidentally, by the end of the year, the VirtualBrainLab had been used by 563 teachers and 1,070 schoolchildren from all parts of Germany. And it received a prize: the Institute for Education and Media of the Society for Education, Information and Media (Gesellschaft für Pädagogik, Information und Medien e. V.) awarded it the Comenius EduMedia seal in the category “Didactic Digital Media Products” for school and university education.

Plants live and in colour were also presented to students in the shape of the “Pet Plant Project”, as an addition to virtual course contents. Its intention was to give those with more of a connection to animals an understanding not only of their own house plants but also of botany in general. From planting seeds to ripening fruits, trainee teachers were able to accompany and “get to know” their “house plant” over the course of a semester – a learning model that some teaching degree students want to transfer in a modified form into everyday school life.
Uncertainty has its appeal
Simulating biology lessons is also possible outdoors – on the bee trail, in a field of wildflowers, down by the university’s own pond or a stream, Urselbach, which is within walking distance, where the students should then catch the bees with a fishing net and identify them. But the live organisms discovered in the stream were not always on the syllabus and could therefore not always be identified immediately. “This makes some students uncertain and can also bring lecturers out in a cold sweat,” explains Professor Volker Wenzel, professor for didactics of biology. “But we’ve noticed that this uncertainty also makes them creative.” Those happy to work outdoors expand their knowledge of animal and plant species more quickly, he says. Identification apps such as iNaturalist or Flora Incognita can help teaching staff and students alike – “especially since we have familiarised ourselves in greater depth with the possibilities offered by the apps during the pandemic and can, for example, design our own projects with students,” says Wenzel. “We should expose ourselves far more often to first-hand experiences with living organisms.”
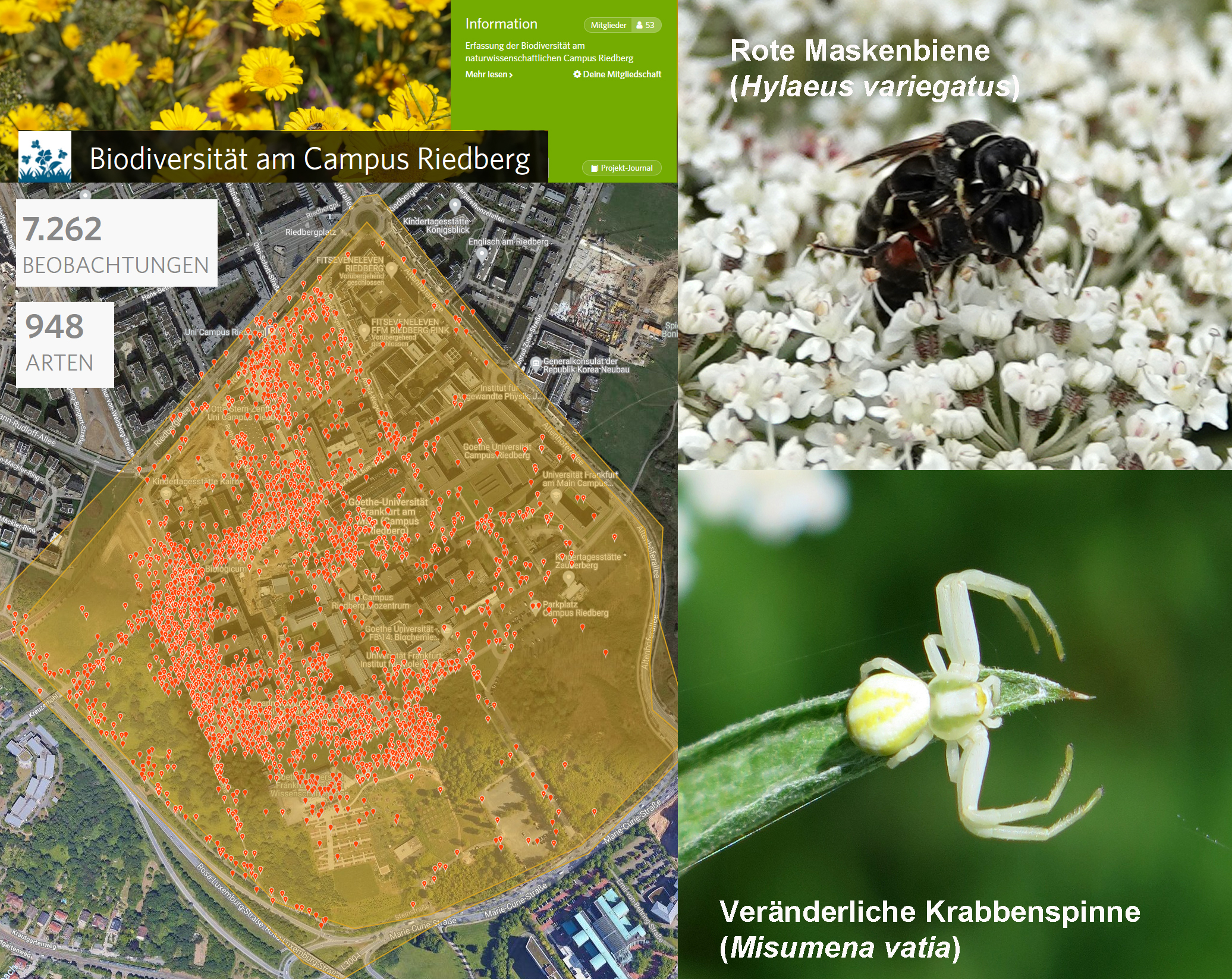
A highlight of the biology degree programme for prospective teachers is the field trip over several days, which can take them to Helgoland or Altmühl Valley, for example. The objective: to expand their knowledge of species, get to know experiential learning concepts and, finally, learn how to plan such trips themselves. In 2020, the usual trips dwindled to a visit to Riedberg Campus – where the iNaturalist biomonitoring project really made history: within a few weeks, 948 different plant, animal and fungal species on Riedberg Campus were identified and mapped. The following year, one-day field trips were already possible – to the Messel Pit Fossil Site, a UNESCO World Heritage Site, for example, or to the nature park in the Taunus and to Weilburg Zoo. In 2022, Helgoland will again be the destination for Professor Wenzel. “We’re very much looking forward to learning together again,” says Wenzel. And as for the appeal of biology outdoors and the experience of being able to learn surprising things in uncertain terrain – “We can’t wait!”
Unser Forschungsprofil: Raum für gute Antworten
Mit sechs Profilbereichen will die Goethe-Universität ihre Kompetenzen stärker bündeln.

