Biochemist and physician Dr Leif S. Ludwig (40) from the Berlin Institute of Health at Charité (BIH) and the Max Delbrück Center will receive the 2023 Paul Ehrlich and Ludwig Darmstaedter Prize for Young Researchers, as the Scientific Council of the Paul Ehrlich Foundation announced today. Building on the latest technologies for the gene sequencing of single cells, prize winner Ludwig has developed a method that can analyse the lifelong regeneration of cells in human blood in a way that is up to 1,000 times quicker, more reliable and less expensive than has previously been possible. In so doing, he is enabling medicine to determine for the first time and with reasonable effort the activity of single blood stem cells in humans.
Our blood renews itself constantly. Each second, millions of new cells are added to our bloodstream which replace dying blood cells. They originate from haematopoietic (blood-forming) stem cells in the bone marrow and then gradually mature over several stages. A distinction is traditionally made between four major developmental trajectories: the first trajectory produces the red blood cells that transport oxygen, the second supplies the thrombocytes, or platelets, that stop bleeding and allow wounds to heal. In the third trajectory, the white blood cells develop, which give us our innate immune defence, such as the granulocytes, for example, and in the fourth, the B and T cells develop, which form the basis for our acquired immune defence in the event of infection. However, as research progressed, the more and more difficult it became to distinguish these trajectories from each other.
Haematopoietic stem cells were discovered in 1961. This discovery enabled the introduction in the 1970s of bone marrow transplants to treat certain types of leukaemia. Observing how transplanted cells behave in the recipient’s organism led to many new insights into haematopoiesis. However, the fact that these insights were obtained under artificial conditions limited their informational value. After all, the transplanted stem cells had been taken beforehand from their natural context. With the help of genetic markers, however, since the 1980s it has been possible to study the development of blood cells in their natural context. This method, called lineage tracing, was applied with ever greater precision over the following decades – but only in animal experiments because, as it goes without saying, inserting artificial genetic markers into humans is out of the question.
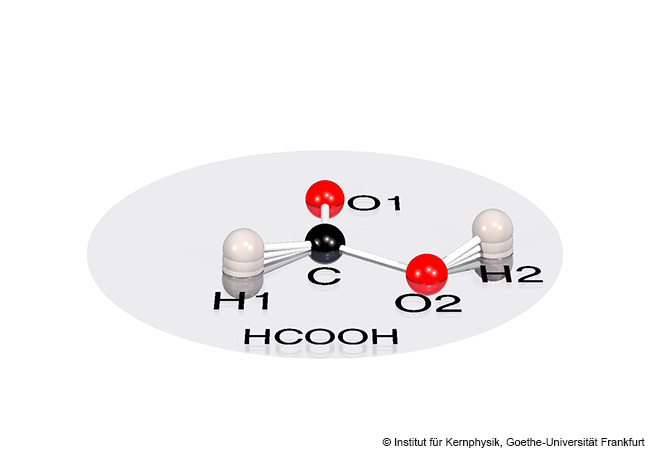
In human blood, lineage tracing is only possible by observing natural DNA mutations that occur after cell division in one daughter cell but not in the other, and which thus only propagate in certain cell families (clones). In the 2010s, researchers attempted to trace such mutations in the entire genome of blood cells. However, in view of the over three billion “letters” (base pairs) in our genome and despite state-of-the-art methods, this is very expensive and prone to error. That is why Leif Ludwig concentrated on evidencing natural mutations in the mitochondria of blood cells. These cellular powerhouses have their own, much smaller genome of around 16,600 base pairs. Leif Ludwig combined their analysis with the latest single-cell sequencing technologies (single-cell omics), which enabled him to make statements about the actual health status of the cells under examination at the same time. He and his team have meanwhile refined their method in such a way that they can analyse tens of thousands of cells in bone marrow and blood samples from a patient.
It has been presumed for a long time that haematopoietic stem cells are not a uniform source but rather form a heterogeneous pool, from which various developmental trajectories develop and branch out in many directions during the continuous formation of new blood. For example, one stem cell might produce only thrombocytes, or platelets, another all kinds of blood cells. The relationships in our blood are therefore highly unclear. Leif Ludwig’s analytical method now makes it possible to disentangle them more easily in order to identify, for example, at which branch point a leukaemia cell develops or a degenerative change occurs. It opens up the possibility for human medicine to conduct such studies in the future for the first time in everyday clinical practice and to derive therapeutic interventions from them.
From 2003 onwards, Dr Leif Si-Hun Ludwig first studied biochemistry at the Free University of Berlin, then human medicine at Charité – Universitätsmedizin Berlin. As a doctoral candidate in biochemistry, he conducted research at the Whitehead Institute of Biomedical Research from 2011 to 2015 and as a postdoctoral researcher at the Broad Institute of MIT and Harvard from 2016 to 2020, both in Cambridge/USA. He has led an Emmy Noether Junior Research Group at the Berlin Institute of Health at Charité and the Berlin Institute for Medical Systems Biology (Max Delbrück Center) since November 2020.
The prize will be awarded – together with the main prize for 2023 – by the Chairman of the Scientific Council of the Paul Ehrlich Foundation on 14 March 2023 at 5.00 p.m. in Frankfurt’s Paulskirche.