Research team led by Goethe University shows that due to quantum physical effects even “flat” molecules are always three-dimensional
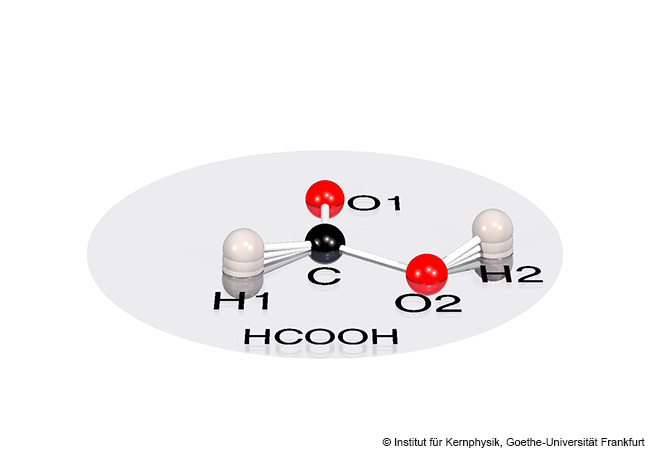
Formic acid is considered a molecule in which all atoms lie in a single plane. A research team at Goethe University, together with cooperation partners, has now demonstrated experimentally that the atoms in formic acid jitter out of this plane continuously on a minimal scale. As a result, the molecule is not flat most of the time but three-dimensional, thereby losing its symmetry. The quivering of the atoms are a a quantum physical effects, according to which particles are never at rest.
Traditional chemistry textbooks present a tidy picture: Atoms in molecules occupy fixed positions, connected by rigid rods. A molecule such as formic acid (methanoic acid, HCOOH) is imagined as two-dimensional – flat as a sheet of paper. But quantum physics tells a different story. In reality, nature resists rigidity and forces even the simplest structures into the third dimension.
Researchers led by Professor Reinhard Dörner of the Institute for Nuclear Physics at Goethe University have now determined the precise spatial structure of the “flat” formic acid molecule using an X-ray beam from the PETRA III synchrotron radiation source at the DESY accelerator center in Hamburg. They collaborated with colleagues from the universities of Kassel, Marburg and Nevada, the Fritz Haber Institute, and the Max Planck Institute for Nuclear Physics.
To do so, they made use of two effects that occur when X-ray radiation strikes a molecule. First, the radiation ejects several electrons from the molecule (photoelectric effect and Auger effect). As a result, the atoms become so highly charged that the molecule bursts apart in an explosion (Coulomb explosion). The scientists succeeded in measuring these processes sequentially, even though they take place within femtoseconds—millionths of a billionth of a second.
For this purpose, they used an apparatus invented at Goethe University and continuously refined since then: The COLTRIMS reaction microscope. Based on the measurement data, they were then able to calculate the original geometry of the formic acid molecule. The result: The two hydrogen atoms of formic acid oscillate slightly back and forth, meaning that the molecule is not flat.
Reinhard Dörner explains: “In the quantum world, atomic nuclei are not tiny spheres that remain fixed in place. They are more like vibrating clouds. Even if we cool a molecule down to absolute zero, this trembling – the so-called zero-point motion – never stops.”
The consequence is radical: An atomic nucleus does not have an exact location, only a probability of being found in a particular place. In a sense, it is “a little bit everywhere”. As a result, a formic acid molecule is effectively three-dimensional at almost every moment.
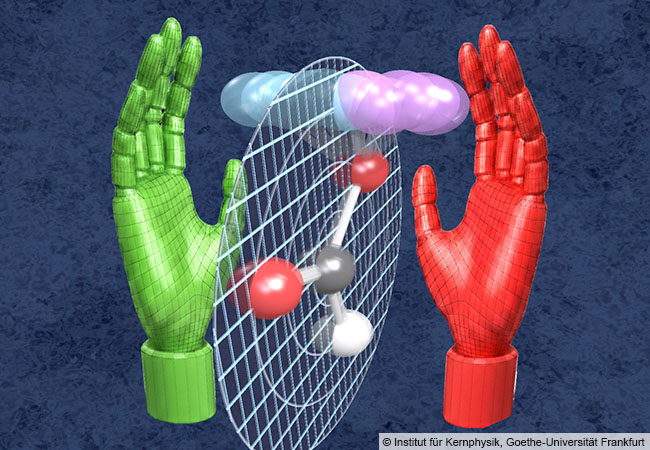
Dörner adds: “Through this tiny step into the third dimension, the molecule loses its symmetry and can no longer be superimposed onto its mirror image – similar to our left and right hands. Formic acid is chiral – it has a left-handed form half the time and a right-handed form the other half.”
In chemistry, two such chiral forms – so-called enantiomers – can have completely different effects: While one form of a molecule may act as a medicine, its mirror image may be ineffective. Normally, this handedness arises from the fixed structure of a molecule.
Dörner concludes: “As we were able to show using the example of formic acid, quantum trembling alone can generate two different mirror-image realities from a symmetrical molecule. This means that handedness – an important property of life – does not arise here from the molecule’s static blueprint, but solely from the incessant trembling in the quantum world. More generally, our findings with formic acid show that geometry is not a static property but a dynamic event, and that a flat molecule is in reality only the average value of its atoms trembling in all directions.”
Publication: D. Tsitsonis, M. Kircher, N. M. Novikovskiy, F. Trinter, J. B. Williams, K. Fehre, L. Kaiser, S. Eckart, O. Kreuz, A. Senftleben, Ph. V. Demekhin, R. Berger, T. Jahnke, M. S. Schöffler, R. Dörner. Probing Instantaneous Single-Molecule Chirality in the Planar Ground State of Formic Acid. Physical Review Letters (2026)