For solid tumors to grow efficiently, they generally need the help of non-transformed, endogenous cells around them. Through the communication of these cells between each other, networks form in the tumor’s environment which stimulate growth. With the support of the Wilhelm Sander Foundation, researchers at Goethe University Frankfurt have now examined such networks. In the process, they discovered that these networks are very resistant to intervention, but the team also succeeded in identifying possible weak points.
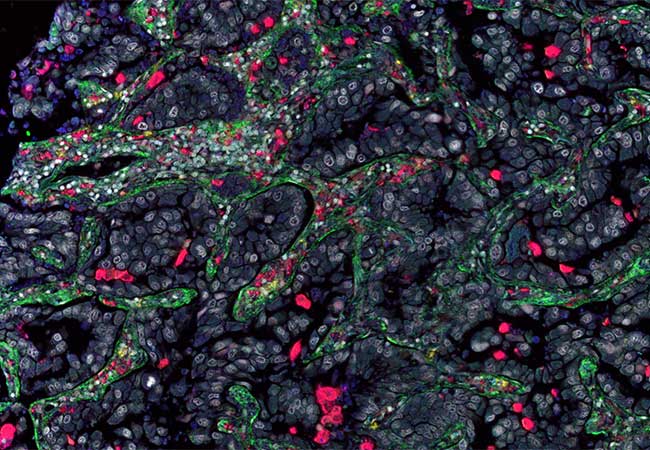
Tumors consist of both the actual, malignant cancer cells and healthy, non-transformed cells in the immediate environment. These include, among other things, the endogenous scavenger cells of the immune system, called macrophages, as well as types of cells that form connective tissue, such as fibroblasts. Both macrophages and fibroblasts normally contribute to keeping tissue in its original healthy state and to restoring its structure after minor or major damage. These capabilities also play an important role in defending the body against the proliferation and spread of cancer cells.
However, cancer cells have developed strategies to reprogram both macrophages and fibroblasts into tumor-promoting cells. In this process, the fibroblasts are altered in such a way that they change the tissue structure so that it helps the tumor cells to survive and spread. For example, if metastases form in the lung, the fibroblasts in the lung are activated first. Macrophages secrete growth and survival factors, which the tumors use, for example, to give themselves a better supply of nutrients and oxygen.
It has long been assumed in cancer research that the deactivation of specific, non-transformed types of cells might be enough for therapy to be successful. However, despite the promising results achieved in research such strategies have so far hardly been successful in the treatment of patients.
A research team led by Professor Andreas Weigert and Professor Bernhard Brüne from Goethe University Frankfurt has now identified possible reasons for this. For their analyses, the researchers used genetically modified mice that spontaneously develop tumors in their breast tissue. Through further genetic modifications, a fat-like molecule produced by the macrophages and released into the tumor environment, the hormone prostaglandin E2, was deactivated in the mammary carcinoma of these mice. Prostaglandin E2 was previously believed – on the basis of cell culture experiments – to have above all tumor-promoting properties. As expected, deactivating prostaglandin E2 also inhibited the growth of mammary carcinoma in the mice. To the surprise of the research team, however, tissue analyses showed that the fibroblasts divided extensively and were activated, and at the same time more metastases developed in the lungs of the mice.
In further trials, the transcriptome of the fibroblasts was analyzed, that is, all the genes read from the genome at that point in time. The researchers were able to show that prostaglandin E2 keeps the fibroblasts in mammary carcinoma in an inactive state by means of a previously unknown signaling pathway, which explains why removing the molecule in the mice led to increased metastasis. The process is evidently similar in humans: Fibroblasts activated in a similar way were also found in the breast tumors of some patients, and these patients were far less likely to survive.
In the course of their histological study of mammary carcinoma, the researchers also encountered a subgroup of macrophages which, similar to fibroblasts, produce parts of the extracellular matrix (the connective tissue between the cells) – above all collagens. Such macrophages, called fibrocytes, were already known from fibrotic disorders (pathological proliferation of connective tissue) of the lung, but their role in tumors was unclear.
That is why the researchers in Frankfurt, together with Professor Rajkumar Savai from the Max Planck Institute for Heart and Lung Research in Bad Nauheim, examined the role of fibrocytes in lung tumors by systematically deactivating them during tumor growth. By means of single-cell sequencing, they were able to corroborate, among other things, that these cells are a key population which coordinates both the growth of the tumor cells and their supply with blood vessels as well as the tumor-promoting activation of other macrophage subtypes.
“The results of our studies illustrate that there are many types of cells in the tumor microenvironment that promote tumor survival, growth and spread in a similar way. The tumor uses central molecular hubs through which it simultaneously reprograms various endogenous cells into tumor promoters. If we want to fight cancer effectively, we need to advance the detection and therapeutic use of such hubs,” says Weigert, summarizing the study results, which were published in the renowned journals Cancer Research and Nature Communications. Identifying such hubs will be a research priority for the participating laboratories in the future.
Publications:
1) E. Strack, P.A. Rolfe, A.F. Fink, K. Bankov, T. Schmid, C. Solbach, R. Savai, W. Sha, L. Pradel, S. Hartmann, B. Brüne, A. Weigert. Identification of tumor-associated macrophage subsets that are associated with breast cancer prognosis. Clin Transl Med (2020), 10:e239. https://doi.org/10.1002/ctm2.239
2) E. Elwakeel, M. Brüggemann, J. Wagih, O. Lityagina, M.A.F. Elewa, Y. Han, T. Froemel, R. Popp, A.M. Nicolas, Y. Schreiber, E. Gradhand, D. Thomas, R. Nüsing, J. Steinmetz-Späh, R. Savai, E. Fokas, I. Fleming, F.R. Greten, K. Zarnack, B. Brüne, A. Weigert. Disruption of prostaglandin E2 signaling in cancer-associated fibroblasts limits mammary carcinoma growth but promotes metastasis. Cancer Res. (2022), 82(7):1380-1395. https://doi.org/10.1158/0008-5472.can-21-2116
3) A. Weigert, X. Zheng, A. Nenzel, K. Turkowski, S. Günther, E. Strack, E. SiraitFischer, E. Elwakeel, I.M. Kur, V.S. Nikam, C. Valasarajan, H. Winter, A. Wissgott, R. Voswinkel, F. Grimminger, B. Brüne, W. Seeger, S. Savai Pullamsetti, R. Savai. Fibrocytes boost tumor-supportive phenotypic switches in the lung cancer niche via the endothelin system. Nat Commun. (2022), 13:6078 https://doi.org/10.1038/s41467-022-33458-8