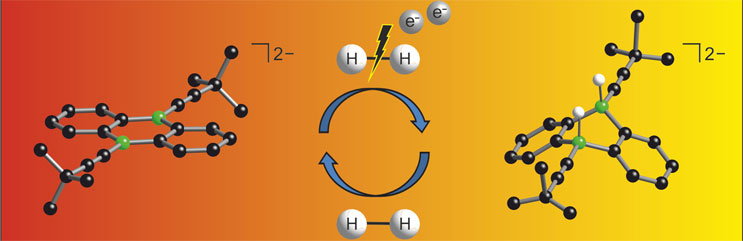
Hydrogen (H2) is an extremely simple molecule and yet a valuable raw material which as a result of the development of sophisticated catalysts is becoming more and more important. In industry and commerce, applications range from food and fertilizer manufacture to crude oil cracking to utilization as an energy source in fuel cells. A challenge lies in splitting the strong H-H bond under mild conditions. Chemists at Goethe University have now developed a new catalyst for the activation of hydrogen by introducing boron atoms into a common organic molecule. The process, which was described in the Angewandte Chemie journal, requires only an electron source in addition and should therefore be usable on a broad scale in future.
The high energy content of the hydrogen molecule meets with a particularly stable bonding situation. It was Paul Sabatier who in 1897 detected for the first time that metals are suitable catalysts for splitting the molecule and harnessing elementary hydrogen for chemical reactions. In 1912 he was awarded the Nobel Prize for Chemistry for this important discovery. The hydrogenation catalysts mostly used today contain toxic or expensive heavy metals, such as nickel, palladium or platinum. Only ten years ago non-metal systems based on boron and phosphorous compounds were discovered which allow comparable reactions.
“My doctoral researcher, Esther von Grotthuss, has achieved yet another major simplification of the non-metal strategy which requires only the boron component”, says Professor Matthias Wagner from the Institute of Inorganic and Analytical Chemistry of Goethe University Frankfurt. “What we additionally need is just an electron source. In the laboratory we chose lithium or potassium for this. When put into practice in the field, it should be possible to substitute this with electrical current.”
In order to explain the intricacies of hydrogen activation above and beyond experimental findings, quantum chemical calculations were carried out in cooperation with Professor Max Holthausen (Goethe University Frankfurt). Detailed knowledge of the reaction process is very important for the system’s further expansion. The objective lies not only in replacing transition metals in the long term but also in opening up the possibility for reactions which are not possible with conventional catalysts.
The chemists in Frankfurt consider that especially substitution reactions are highly promising which permit easy access to compounds of hydrogen with other elements. Expensive and potentially hazardous processes are still mostly used for such syntheses. For example, the simplified production of silicon-hydrogen compounds would be extremely attractive for the semiconductor industry.
[dt_call_to_action content_size=“small“ background=“fancy“ line=“true“ animation=“fadeIn“]
Publication: E. von Grotthuss et al: Reversible Dihydrogen Activation by Reduced Aryl Boranes as Main-Group Ambiphiles, in: Angew. Chem. Int. Ed. 2016, DOI: 10.1002/anie.201608324
[/dt_call_to_action]
Source: Press Release 21/10/16