Bacteria produce a cocktail of various bioactive natural products in order to survive in hostile environments with competing (micro)organisms. In the current issue of Nature Chemical Biology, researchers at Goethe University demonstrate that they do so by modifying basic structures, similar to the approach taken in pharmaceutical research.
Phenazines are widespread and chemically diverse bacterial natural products that can fulfil various biological functions. Like antibiotics, some derivatives kill bacteria; others are toxic to fungi and/or cancer cells. There are also derivatives that allow the bacteria to survive in an environment that is hostile to them, such as the human body. These virulence factors are often crucial for the bacteria to become pathogenic.
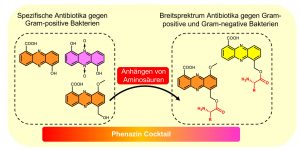
Biochemically, all phenazines are derived from simple basic structures, such as the phenazine-1,6-dicarboxylic acid or phenazine-1-carboxylic acid, whose biosynthesis is well understood. However, these initial structures can be drastically modified in the periphery so that a large number of phenazine derivatives are possible, several of which can indeed be found in different bacteria. The Molecular Biotechnology research group led by Professor Helge Bode has now succeeded in identifying new mechanisms that allow the bacteria to modify these simple basic structures, resulting in derivatives that act on both Gram-positive and Gram-negative bacteria, as well as on cells of higher organisms.
“Bacteria are able to determine which derivatives should be created using a central new aldehyde intermediate as well as the activation of a second biosynthetic gene cluster,” explains Dr. Yi-Ming Shi, who investigated this system during a Humboldt scholarship. This means that bacteria use mechanisms for drug development similar to those used in pharmaceutical research, where new derivatives are produced using the same basic structures. Most likely the bacteria use the phenazines to kill other bacteria and fungi that are food competitors in their particular ecosystem. Using a strategy of creating many different kinds of derivatives, the bacteria are well equipped to counteract unknown competitors, as the cocktail of derivatives exhibits a wide range of biological activity.
“It would now be fascinating to find out how bacteria actually recognise which derivatives are required at a given time,” states Helge Bode. “Either they produce only those derivatives that are actually required, or the bacteria keep an arsenal of derivatives so that they are prepared for any situation.”
The group will therefore continue their research in this area. First results on the underlying regulation mechanisms that could also be used for biotechnical applications appear promising.
[dt_call_to_action content_size=“small“ background=“fancy“ line=“true“ animation=“fadeIn“]
Publication:
Yi-Ming Shi, Alexander O. Brachmann, Margaretha Westphalen, Nick Neubacher, Nicholas J. Tobias, Helge B. Bode, Dual phenazine gene clusters enable diversification during biosynthesis. Nature Chemical Biology, 2019, 10.1038/s41589-019-0246-1.
[/dt_call_to_action]