Two scientists at Goethe University Frankfurt are seeking weaknesses in sophisticated bacterial defense systems. Their goal: Find new approaches to combat bacterial infections – something they will also be focusing on as part of the SCALE cluster initiative.
Transport proteins are membrane proteins that perform the special task of ensuring that substances can pass between the cell’s interior and the extracellular space. One large family of these transporters is known as ABC transporters, whereby ABC stands for ATP-binding cassettes. Their characteristic feature is that they split energy-carrying adenosine triphosphate (ATP) molecules to release the energy they need to actively transport substances through the cell membrane. This characteristic classifies them as part of the family of primary active transporters.
Clemens Glaubitz from Goethe University’s Institute of Biophysical Chemistry is researching ABC proteins that regulate transport in bacteria. He specializes in Gram-negative bacteria, which include Escherichia coli, Klebsiella and Acinetobacter baumannii, and which possess not only one membrane surrounding and protecting the cell contents – the cell membrane – but also have a second outer membrane separated from the inner one by the periplasm. The two membranes are constructed differently: whereas the inner membrane comprises a symmetrical lipid bilayer, the outer membrane is asymmetrical and contains complex compounds consisting of fatty acids and lipopolysaccharides (LPS), i.e. polysaccharides. “These are the most important components of the outer membrane. They help maintain the bacteria’s cellular architecture,” Glaubitz explains.
SCALE – Subcellular Architecture of Life
Cells consist of billions of molecules, organized as individual molecules, large molecular complexes or whole organelles. While the functions of many individual molecules are known, in many cases it remains unclear how the architecture inside a cell arises and functions, and how its various parts interact. To gain a better understanding of how cells really function and how their various “machines” cooperate, the scientists at SCALE want to discover the principles of cellular self-organization and create a simulation of the cell in high spatial and temporal resolution. On February 2, 2024, the German Research Foundation (DFG) announced that, based on its draft concept, SCALE may submit a full proposal in August 2024 for funding as a Cluster of Excellence from 2026. https://scale-frankfurt.org
However, for LPS to fulfill their stabilizing function, they first have to actually arrive at the outer membrane. This vital task falls to the ABC transporters, which bring the polysaccharides from the cell’s interior, where they are produced, through the cell membrane and the periplasm, to their destination on the outer membrane. Glaubitz’ lab is currently investigating the ABC transporter MsbA and the transport system linked to this protein, which creates a kind of bridge from the inner membrane to the outer one – which the polysaccharides just have to flow across in the outer membrane’s direction. Glaubitz intends to use Escherichia coli, a bacterium that lives in the gut, to find out how the whole process is controlled.
New targets for antibiotics
The experimental method applied by his team is known as solid-state NMR spectroscopy. Whereas nuclear magnetic resonance spectroscopy usually requires the samples under investigation to be soluble, when the technique is expanded to non-soluble samples, we speak of solid-state NMR spectroscopy. The researchers in Glaubitz’ lab discovered that MsbA – and its related ABC transporters in Gram-negative bacteria – seem to adapt the transportation of polysaccharides to changes in the environment. This means that the LPS transport system can become more efficient as and when necessary. Glaubitz plans to use MsbA as a model protein for all other such systems in fellow Gram-negative bacteria, including Acetinobacter baumannii. Asked where this basic research could lead to, he says that in the future, LPS-transporting proteins like MsbA could become new targets in the treatment of bacterial infections using antibiotics. “Maybe one day we’ll be able to inhibit the proteins involved in transporting LPS.” Unable to reach the outer cell membrane, they would no longer be able to stabilize the bacterial architecture.
The antibiotic efflux pump Klaas Martinus Pos is researching also appears to determine the correct construction of the bacterial cell envelope. The architecture of the cell envelope must be intact for bacteria to be resistant to antibiotics. This is why, as part of his work at SCALE, Pos is working together with other working groups to examine a number of bacterial cell-envelope architectures. “This is an exciting area about which we don’t know very much yet. Maybe we’ll uncover new ways of combating antibiotic-resistant bacteria in the future.” Also as part of SCALE, Clemens Glaubitz is investigating the transport proteins that help maintain the double membrane in Gram-negative bacteria. He is focusing on bacterial lipid vesicles discharged from the outer membrane, which appear to be directly connected with its resistance and stress response mechanisms. “In addition, we’re making our experience in solid-state NMR available to SCALE – a method that has proven its worth when it comes to analyzing proteins and lipids in membranes.”
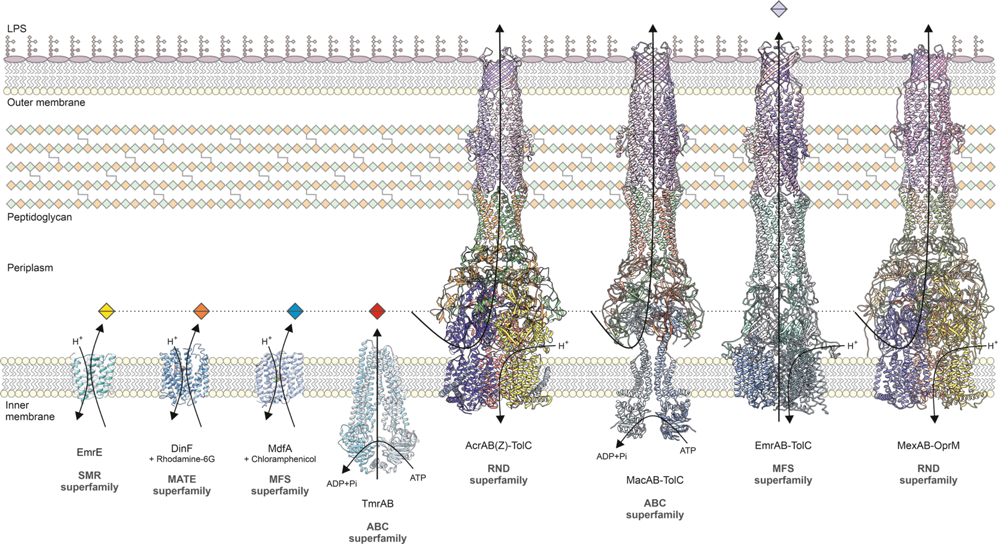
There is another reason why preventing the transport of polysaccharides outside the cell would be important for combating pathogens: LPS also form an initial external barrier against antibiotics. They do so because the polysaccharides are hydrophilic, i.e. attracted to water, while antibiotics are hydrophobic and therefore attracted to fats. This is why the latter generally cannot permeate the outer membrane. If they do manage to cross it, they are immediately met by a second line of defense, which Klaas Martinus Pos is investigating at Goethe University’s Institute of Biochemistry. He focuses on the RND family of transporters, whereby RND stands for resistance-nodulation division. Embedded in the bacteria’s inner cell membrane, these transporters belong to the family of secondary active transporters, as they use the electrochemical gradients of ions to supply the energy needed to drive active transport.
Pumps of this type act like sentries that prevent antibiotics from diffusing through the inner cell membrane into the cytoplasm. Any intruding molecules are tackled before they get that far, and are exported out of the cell and back into the environment. During this process, the ABC transporters use what is known as the antiport mechanism, Pos explains: “They transport the ions in one direction, the cell’s interior, and the toxic substance in the opposite direction, its exterior.” Po researches Escherichia coli and Klebsiella pneumoniae bacteria, among others, both of which rely on a tripartite efflux pump called AcrAB-TolC, whose operation his lab has discovered: While AcrB is the actual RND transporter, AcrA is an adaptor protein, located in the periplasm, which connects the RND transporter to the third pump component, the pore TolC in the outer membrane. All three together form a long, flexible tunnel system, through which antibiotics are exported out of the cell. “This tripartite efflux pump is hugely efficient. It recognizes almost all known antibiotics and pumps them out.” Scientists also speak of a multidrug efflux pump, which is the sole factor responsible for certain bacteria’s resistance to a large number of antibiotics (a phenomenon known as multi-resistance).
Pos and his team have also discovered how the efflux pump obtains the necessary energy. It binds protons from the periplasm and releases them into the cytoplasm. “The resulting electrostatic changes in the pump’s membrane component ensure that the antibiotic molecules in the tunnel system move in one direction only, to the outside.” The flexible tunnel mechanism resembles a peristaltic pump like the one found in our esophagus, which enables us to eat solid food even while standing on our heads: The food we swallow arrives in the stomach despite the opposing force of gravity. The bacterial efflux pump uses the same mechanism to prevent the antibiotics from sliding back into the cell during the expulsion process.
Snapshots of the efflux pump
Finding out where and how antibiotics are intercepted and expelled requires high-resolution structures, which – in the ideal case – make individual atoms visible. Pos’ lab deploys specially grown crystals to create electron density maps, which are then used to build 3-D structures of the tripartite pumps – a process that can also be done using single-particle cryo-electron microscopy. “Both methods supply snapshots, individual images of the efflux pump at work, which we can view one after the other. It’s like a flipbook – we can actually observe the antibiotic being pumped through the tunnel.”
Working together with Prof. Achilleas Frangakis’ working group and the Institut Pasteur in Lille, Pos’ team used snapshots to develop new kinds of inhibitors, i.e. substances that specifically hinder the multi-drug efflux pump. “This could enable us to once again effectively fight bacterial infections using existing antibiotics to which bacteria have already exhibited resistance.” The effects of the inhibitors were recently demonstrated in a pulmonary model in mice infected with Klebsiella pneumoniae. In December 2023, Clara Börnsen and Reinke Müller, two researchers from Pos’ and Frangakis’ working groups, were awarded Goethe University’s Unibator Innovation prize for their work on these inhibitors as part of the “Antibiotics reloaded” project.
Basic research into bacterial resistance mechanisms is urgently needed, Pos says. “At present, 1.3 million people around the world are killed by multi-resistant pathogens every year. If we don’t act now, by 2050 we can expect ten million deaths a year.” That would make multi-resistant pathogens even deadlier than cancer is today.
Author: Andreas Lorenz-Meyer













