Hydrogen storage suitable for everyday use and the prospect of breaking down carbon dioxide
The discovery was almost too good to be true: In 2013, members of the research group of Volker Müller, head of the Department of Molecular Microbiology and Bioenergetics at Goethe University, found a remarkable enzyme: the “hydrogen-dependent CO2 reductase” – also known by its abbreviation HDCR. HDCR is the only known enzyme that processes nothing but the gases hydrogen (H2) and carbon dioxide (CO2), transforming them into formic acid. In other words, HDCR stores hydrogen in the form of formic acid, which has a very convenient property: it is liquid. Compared to the extremely explosive gas H2, this makes it much easier and less risky to store, transport and use hydrogen to generate energy – a precondition to realistically using hydrogen as a CO2-neutral energy carrier of the future in everyday life.
The word “reductase” in the enzyme’s name indicates that producing formic acid from H2 and CO2 changes the electronic structure of the atoms/molecules involved: The hydrogen passes electrons on to a CO2 molecule. No other transfer molecules are involved in this electron transaction. There are two additional properties that set the HDCR enzyme apart: Firstly, the process of formic acid production is completely reversible, meaning the hydrogen stored in this way ultimately can be released again and can serve as an energy carrier once more.
Secondly, the HDCR enzyme accelerates formic acid production in an extremely efficient manner: “The rates at which CO2 and H2 react to formic acid and back under HDCR influence are the highest ever measured,” Müller reports, adding that, “they are many times greater than is the case for other biological or chemical catalysts.” Whereas the use of chemical catalysts requires expensive precious metal catalysts as well as extreme conditions such as great pressures and high heat, formic acid production using HDCR requires neither, he says.
Inconspicuous, but crucial
The HDCR enzyme is found in the cytoplasm of various bacteria, including in the heat-loving bacterium Thermoanaerobacter kivui (T. kivui), which lives in the absence of air in the mud of the East African Lake Kivu, for example, as well as in Acetobacterium woodii (A. woodii), which prefers moderate temperatures. However, when Müller and members of his research group began studying HDCR in the laboratory ten years ago, they discovered an inconspicuous but crucial detail hindering its use in practical formic acid production (and thus the storage of hydrogen): HDCR is extremely sensitive to oxygen. Even the tiniest amounts of oxygen mean the enzyme can no longer perform its task in formic acid production.
The bioreactor Fabian Schwarz has now developed as part of his doctoral thesis in Müller’s team circumvents this problem in a way that is both simple and elegant: In it, the HDCR enzyme is not isolated from the bacteria, but left in its “natural environment”, i.e. the cytoplasm of the bacteria. “We are introducing our bioreactor in ‘Joule’, a respected journal for chemical and physical engineering,” Müller says, “and we demonstrate how it ran very stably for more than two weeks.” The fact that the paper by the Frankfurt microbiologists was accepted for publication in “Joule” constitutes proof that the process is suitable for everyday use, he adds.
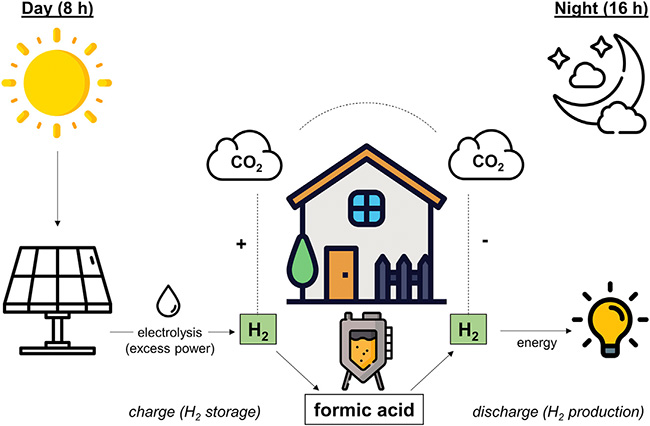
As part of this process, the bacteria in the bioreactor are supplied with hydrogen and carbon dioxide during an eight-hour “day phase”, allowing them to form formic acid with the help of the HDCR enzyme, or – put differently – enabling them to store the hydrogen in the form of formic acid. During the ensuing 16-hour “night phase”, the bacteria are cut off from the hydrogen supply and completely release the hydrogen again.
Hydrogen from renewable sources
The designations “day” and “night” already indicate that the reactor does not run with just any kind of hydrogen, but with sustainably produced, i.e. “green” hydrogen. The H2 is produced from water (H2O) by means of electrolysis, i.e. by “energizing” the water. In the case of green hydrogen, this electricity comes from renewable sources, typically from solar cells in photovoltaic systems. Ergo: The hydrogen is available during the day – “precisely when the sun is shining,” Müller comments.
The formic acid, however, does not form on its own: It normally reacts further to form acetic acid, which – unlike formic acid – cannot simply be converted back into the gaseous starting products hydrogen (H2) and carbon dioxide (CO2). The subsequent reaction to acetic acid would therefore result in some of the stored hydrogen being lost to energy production. To prevent this from happening, the researchers operated the bioreactor with genetically modified A. woodii bacteria in which acetic acid formation is suppressed.
A characteristic feature of the bioreactor developed by Schwarz and Müller is that both reaction directions – the formation of formic acid and the release of hydrogen – take place in the same environment, a fact that is particularly useful if municipal energy suppliers or private households want to use the process “at home”. Theoretically, the reactor even removes carbon dioxide from the air during the day phase. However, since this is released again over the course of the bioreactor’s operation (during the night phase), the atmosphere’s CO2 content remains the same.
In light of this, the net removal of carbon dioxide from the atmosphere appears to be more than just a pipe dream or science fiction – all it takes is further research from Müller’s team. After discovering the HDCR enzyme and showing in initial investigations that even the smallest amounts of (atmospheric) oxygen suffice to render it non-functional, they not only started developing the bioreactor, i.e. a potential (important!) application that simply circumvents the problems caused by the influence of oxygen.
Using the HDCR of the T. kivui bacterium discovered in East Africa, the team also investigated the enzyme’s structure in more detail, expecting that it would tell them more about the enzyme’s functioning. One of their first discoveries was that the HDCR enzyme consists of four modules: one where the H2 molecules, which make up gaseous hydrogen, are broken down. Then two small modules, each containing iron and sulfur. And finally, one at which formic acid is formed from carbon dioxide.
“When we found the small iron-sulfur subunits, we knew they must be responsible for conducting electrons from one part of the enzyme to the other during formic acid production,” says Müller, adding that, “this is where the electron transaction happens that is crucial for the enzyme activity of the HDCR reductase.” Nevertheless, Müller, his colleagues and researchers from Marburg and Basel, still dug deep to prove this – an effort for which they now have been rewarded with a publication in the highly renown scientific journal “Nature”. By elucidating the HDCR structure with atomic precision, the researchers found out all the steps in formic acid formation. They determined how the storage of hydrogen in the form of (liquid) formic acid works and why the process is so efficient that its mechanism is suitable as the operating principle of a bioreactor designed for everyday use.
After initially observing the bacteria and their HDCRs with a conventional electron microscope, the team now switched to cryo-electron microscopy: Although this constitutes “simple” electron microscopy, in which the object under investigation is cooled by liquid nitrogen (-196°C) or liquid helium (-269°C), for example, cryo-electron microscopy offers images with atomic resolution – thanks to the more powerful cameras used in this method, the fact that the images are less “blurred” at low temperatures due to the atoms’ and molecules’ own movements, and the complex calculations that supplement the microscope images,
Mysterious filaments
As early as 2016, members of Müller’s research group had observed that HDCR forms long filaments – something very few enzymes do. Müller reports: “We already knew that this filamentous structure is important to the enzyme’s functioning when we examined HDCR from minimally genetically modified bacteria. Their manipulated HDCR, which did not form filaments, exhibited greatly reduced enzyme activity.”
However, the exact role played by the filaments only would be analyzed in detail later with the help of cryo-electron microscopy. Together with scientists from the “Center for Synthetic Microbiology” at Philipps-Universität Marburg, Müller and his team found that the backbone of the filaments consists of the two small HDCR subunits containing iron and sulfur. “Thousands of electron-conducting iron atoms are thus stored together in the filaments, forming a kind of nanowire,” Müller reports.
For the researchers around Müller, the individual “snapshots” of their experiments thus come together in a “film” that is screened in acetogenic bacteria such as T. kivui and A. woodii: “The first module splits the hydrogen (H2), releasing electrons and pumping them into the nanowire,” Müller describes. Within the wire, which consists of the second and third modules, the electrons are transported to the nearest CO2 molecule and passed on to it. Formic acid (HCOOH) is formed under the influence of the fourth module.
“The two partial reactions that take place at the first and fourth modules are thus decoupled. They no longer have to take place simultaneously, because the electrons are temporarily stored in the nanowire, so to speak,” Müller explains. This corresponds precisely to the situation acetogenic bacteria find in nature: If they encounter a bubble of hydrogen, they can “digest” it and temporarily store the released electrons in the filament until a CO2 molecule becomes available. However, this is not always the case, Müller says, adding that the wire therefore holds an ecological advantage because the bacteria can use it to adapt their metabolic processes (H2 splitting, formic acid formation) to the prevailing environmental conditions.
Enzyme filament bundle
Müller says that, together with cell structure biologists from Basel, his team also found that hundreds of the filaments are wound around each other, forming a ring-like structure anchored in the membrane of the bacterial cells. “The formation of the filaments and their bundling increase their concentration in the bacterial cell quite considerably,” he explains. “This means the bacteria are even better adapted to low or fluctuating hydrogen concentrations in their environment.”
Having now discovered how the HDCR forms formic acid (even if some details are still missing), Volker Müller and his team of scientists can now start optimizing the process. “We could, for instance, change the HDCR modules so the bacteria no longer feed on hydrogen but on carbon monoxide, for example,” he says. “Or we could try to make the HDCR more stable – less sensitive to oxygen. Or create a synthetic nanowire that we can use to capture carbon dioxide from the atmosphere.” That is something the bioreactor developed by his (former) graduate students is not yet able to do.
Stefanie Hense